
ESS227
Prof. Jin-Yi Yu

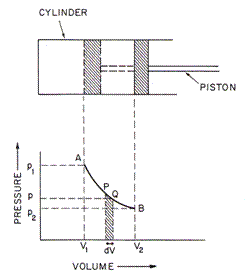
•Therefore, when heat is added to a gas, there will be some combination of an expansion of the gas (i.e. the work) and an increase in its temperature (i.e. the increase in internal energy):
Heat added to the gas = work done by the gas + temp. increase of the
gas
DH = p Da + Cv DT
volume
change of the gas

specific
heat at constant volume

(from Atmospheric Sciences: An
Intro. Survey)