
ESS200A
Prof. Jin-Yi Yu
Why Selective Absorption/Emission?
qRadiation energy is absorbed or emitted to change the energy levels of atoms or molecular.
qThe energy levels of atoms and molecular are discrete but not continuous.
qTherefore, atoms and molecular can absorb or emit certain amounts of energy that correspond to the differences between the differences of their energy levels.
è Absorb or emit at selective frequencies. 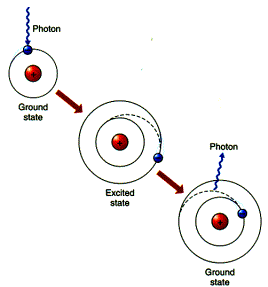

(from Understanding Weather & Climate)


