
ESS124
Prof. Jin-Yi Yu
Saturation
Vapor Pressure
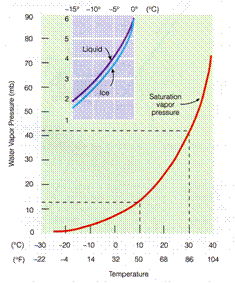
•Saturation vapor pressure describes how much water vapor is needed to make the
air saturated
at any given temperature.
•Saturation vapor pressure depends primarily on the air temperature in
the following
way:
è
•Saturation pressure increases exponentially with air temperature.

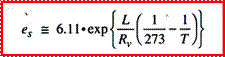

The
Clausius-Clapeyron
Equation
L: latent heat of evaporation; a: specific volume of vapor and liquid