Research in atmospheric aerosols is fueled by the role they play
in air pollution, climate, remote sensing, and chemistry.
The latter has received much attention of late as polar stratospheric
clouds (PSCs) are known to serve as centres for heterogeneous chemistry
which enhance ozone-destroying catalytic cycles. Aerosols also tend to
be very efficient scatterers and absorbers of solar radiation.
They range in size from
![]() m for stratospheric sulphates and cloud condensation nuclei (CCN)
to >1 mm for rain drops and hail. They also vary greatly in number density,
composition and shape, factors which depend on their origin and age.
m for stratospheric sulphates and cloud condensation nuclei (CCN)
to >1 mm for rain drops and hail. They also vary greatly in number density,
composition and shape, factors which depend on their origin and age.
To aid in radiative transfer calculations, analysis, and retrievals,
aerosol models are often employed.
Aerosol models are analytic expressions relating aerosol radius to
number density and usually have one or more adjustable parameters.
A number of `standard' distributions have arisen over the years, each
suited to describing a particular type of aerosol.
Two of these will be used in this study. The first is the
standard gamma distribution
(Deirmendjian, 1969; Hansen and Travis, 1974),
| (3.62) |
Often it is advantageous to express the size parameters of the
different distributions in terms of two common parameters.
This is useful for inter-comparisons between size distributions
as well as in their retrieval.
The area-weighted mean, or effective radius, is used as larger particles
tend to be more efficient scatterers,
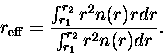 |
(3.63) |
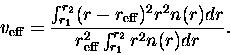 |
(3.64) |
| = | a | (3.65) | |
| = | b | (3.66) |
| = | (3.67) | ||
| = | (3.68) |
Four common types of atmospheric aerosols are discussed below.
Stratospheric Sulphates.
This type of aerosol is present throughout the stratosphere in a
submicron sulphate haze, concentrated mainly between the tropopause
and an altitude of ![]() 30 km. They possess large spatial and temporal
variability (Turco et al., 1982, and references therein).
This is especially true after a large volcanic eruption
where large amounts of SO2, which is quickly oxidized to
H2SO4, can be injected directly into the stratosphere.
30 km. They possess large spatial and temporal
variability (Turco et al., 1982, and references therein).
This is especially true after a large volcanic eruption
where large amounts of SO2, which is quickly oxidized to
H2SO4, can be injected directly into the stratosphere.
Stratospheric sulphates are spherical particles composed of
water and sulphuric acid in the liquid phase.
The general composition of a sulphate aerosol is represented as
(1-x)H2O+xH2SO4, where x is the sulphuric acid weight fraction.
Generally, x is approximately 0.75 (Yue et al., 1994) but this
varies with temperature and water vapour concentration.
The log-normal size distribution is used to represent
stratospheric sulphates. There is also some variability in the
distribution size parameters:
![]() m (Kent et al., 1995)
and
m (Kent et al., 1995)
and
![]() (Brogniez et al., 1997).
After a volcanic eruption,
(Brogniez et al., 1997).
After a volcanic eruption,
![]() may be as large as 0.5
may be as large as 0.5 ![]() m
(Kent et al., 1995).
A typical sulphate size distribution is given in Figure 2.1.
m
(Kent et al., 1995).
A typical sulphate size distribution is given in Figure 2.1.
The particle refractive index varies with temperature, humidity, composition and wavelength (Palmer and Williams, 1975; Steele and Hamill, 1981). The variation with altitude arises mainly from the change in composition. The real and imaginary refractive indices for a 0.25 H2O+0.75 H2SO4 composition are shown in Figure 2.2 (Palmer and Williams, 1975).
Marine Aerosols.
Marine aerosols will be present over water inside the marine boundary
layer. Over the ocean, the principle constituent is sea-salt.
The sea-salt aerosol is produced by the agitation of the ocean surface
by wind (e.g.: Fitzgerald, 1991). Typical composition of the marine aerosol
is 0.30NaCl+0.70H2O by volume.
In general, vertical profiles are strongly dependent upon height,
windspeed, and atmospheric stability (de Leeuw, 1986).
However, for the purposes of this study, it is sufficient to take the
number density as a function of windspeed. An exponential relationship
is adopted (Toba, 1961),
Cumulus Clouds.
Cumulus clouds are formed through convective processes.
A parcel of warm air
near the surface
rises and cools until it becomes supersaturated.
Cloud droplets are largely composed of pure water but
can also have trace amounts of soot or other soluble inorganics.
The vertical extent of a cloud varies greatly from a few hundred
metres for fair weather cumulus up to 14 km for a towering cumulonimbus.
Cloud optical depth are quite variable and can be as large as 1000.
The size of the cloud droplets will depend on both the type of cloud
and its maturity with droplet radii ranging from ![]() m to 100
m to 100![]() m.
Falling raindrops have radii on the order of 1 mm.
Standard gamma size distributions are quite useful for representing
cloud droplet size distributions (Diermendjian, 1969).
The parameters for a cumulus cloud of moderate thickness and mode
radius of 6
m.
Falling raindrops have radii on the order of 1 mm.
Standard gamma size distributions are quite useful for representing
cloud droplet size distributions (Diermendjian, 1969).
The parameters for a cumulus cloud of moderate thickness and mode
radius of 6 ![]() m are given in Table 2.2. The corresponding
size distribution is shown in Figure 2.1.
The real and imaginary refractive indices for water are shown in
Figure 2.2 (Liou, 1992).
m are given in Table 2.2. The corresponding
size distribution is shown in Figure 2.1.
The real and imaginary refractive indices for water are shown in
Figure 2.2 (Liou, 1992).
Cirrus Clouds.
Cirrus clouds, formed near the tropopause, are composed of
ice crystals and supercooled water droplets.
Ice crystals are known to be non-spherical ranging in shape from
hexagonal cross-section columns, hexagonal cross-section plates,
dendrite, needle, or some combination thereof (Rogers and Yau, 1989).
Factors governing their shape include temperature, saturation ratio, and
atmospheric conditions (Liou, 1980). In principle, it is possible
to calculate the scattering properties for any shape of particle; however,
this is generally much more complicated than for spherical particles.
As such, ice crystals will be assumed to follow Mie theory with an
equivalent radius.
The standard-gamma size distribution is adopted with a mode radius
of 25 ![]() m. The real and imaginary refractive indices for ice are
shown in Figure 2.2 (Liou, 1992).
m. The real and imaginary refractive indices for ice are
shown in Figure 2.2 (Liou, 1992).