In 2001, ESS/CGECR researchers Ellen Druffel, John Southon and Susan Trumbore were awarded $2 million by the W.M. Keck Foundation for the development of an accelerator mass spectrometry (AMS) facility – the Keck-Carbon Cycle AMS facility – for radiocarbon measurements in support of carbon cycle research at University of California, Irvine.
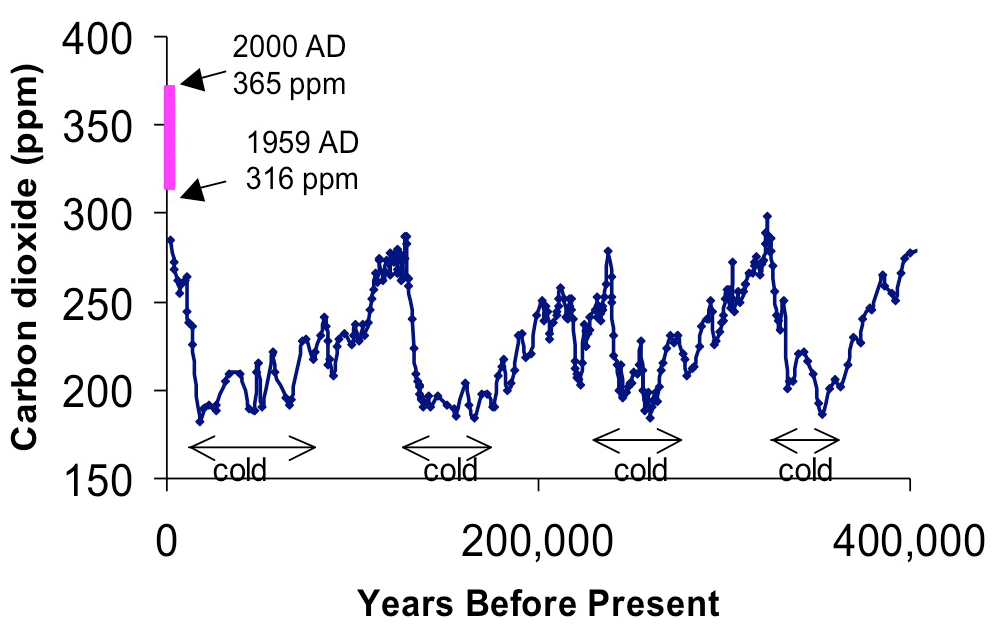
The amount of carbon dioxide in the atmosphere today is higher than that at any time in the last 420,000 years (figure 1). While the roughly 30% increase in CO2 concentration observed over the past 150 years(figure 2) is traceable to human activ ities such as fossil fuel burning and clearing of forests for agriculture, significant changes in CO2 also occurred in the past, presumably related to shifts in global climate. Carbon dioxide in the atmosphere exchanges dynamically with carbon dissolved in oceans and stored in plants and soils on land (figure 3). Changes in atmospheric CO2 clearly must be explained by repartitioning of carbon among these three reservoirs. However, scientists do not yet fully understand the fundamental processes controlling this carbon “cycle”. More research is necessary to explain past changes in CO2 and to predict how CO2, given continued fossil fuel emissions, will change in the future.Radiocarbon (14C), a rare isotope of carbon, is used to determine rates of exchange of carbon between the ocean, land and atmosphere. For exchanges on time scales of less than a human life span, 14C produced by atmospheric weapons testing (between 1953 and 1963), as it dissolves in surface oceans and is taken up and respired by land plants can be traced. On longer timescales, the radioactive decay of 14C provides information on slower exchanges with the much larger stores of carbon in the deep ocean and the carbon stabilized in soils and sediments. Radiocarbon is the best and often the only way to quantify rates of exchange of carbon among reservoirs. This is the key to achieving predictive understanding of the carbon cycle.